Abstract
Background: Cardiac involvement is integral in staging and prognosis of immunoglobulin light chain (AL) amyloidosis. The N-terminal prohormone of brain natriuretic peptide (NT proBNP) is a cardiac biomarker used in screening for cardiac involvement and staging the disease. Transthoracic echocardiogram (TTE) and cardiac magnetic resonance (CMR) are the imaging modalities recommended to determine cardiac involvement and function.
Methods: We conducted a retrospective review of all patients with biopsy proven systemic AL amyloidosis seen at the mayo clinic between Jan 1, 2006 and Dec 30, 2015. The aim of the study is to identify the nature of abnormalities in cardiac biomarkers and echocardiographic features in patients with AL amyloidosis and the ability of these investigations to diagnose cardiac involvement. We first identified all patients with AL amyloidosis that underwent endomyocardial biopsy for suspicion of cardiac involvement (Cohort 1). We then analyzed a cohort (Cohort 2) which consisted of patients who had serum NT proBNP and a comprehensive echocardiographic evaluation at time of diagnosis.
Results: 179 patients with AL amyloidosis underwent endomyocardial biopsy (Cohort 1) of whom 173 had evidence of amyloid deposition. In this cohort, 159 patients had NT proBNP performed at the time of the procedure. The NT proBNP was elevated (>300) in all 159 patients with a median NT proBNP of 4917 (range 355-69541). The median left ventricular ejection fraction (LVEF), interventricular septal (IVS) thickness and strain rate were 54 (range 10-77), 15 (range 8-30) and -9 (range -21 to 0) respectively. CMR findings were consistent or suggestive of light chain amyloidosis in 38/42 patients, yielding a sensitivity of 90 percent. The LVEF, IVS thickness and strain rate were abnormal in 89/168 (53%), 102/64 (61%) and 92/95 (97%) respectively. 95 patients with biopsy proven cardiac amyloidosis had complete echocardiogram data available on LVEF, IVS thickness and strain rate, with 97% (n=92) presenting with an abnormality in at least one of these variables . CMR findings were consistent or suggestive of light chain amyloidosis in 38/42 patients, yielding a sensitivity of 90 percent.
Patients with a normal NT proBNP and normal echocardiogram were considered disease free (true negative), based on our initial analysis of these investigations in Cohort 1.
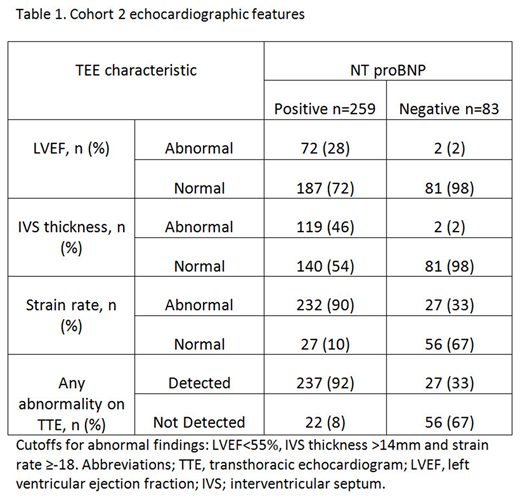
Cohort 2 consisted of 342 consecutive patients. The median NT pro BNP was 1878 (25-48214). The median LVEF, IVS thickness and strain rate were 63 (22-90), 14 (6-25) and -13 (-25 to -3) respectively. 259 (76%) patients had a positive NT proBNP (above 300), of whom 237 (92%) had an abnormality detected on TTE. 83 patients had a negative NT proBNP, of whom 27 (33%) had an abnormality in either LVEF, IVS thickness or strain rate. 19 of these 27 patients had a borderline reduced strain rate between -17 and -18, whilst the remaining 8 patients had a strain between -14 and -15. Only 6/27 patients were considered to have possible early cardiac involvement and none have any other diagnostic or classical features of amyloidosis on TTE.
Conclusion: The combination of NT proBNP and comprehensive echocardiographic evaluation provides substantial information to diagnose cardiac amyloidosis in a significant portion of patients negating the need for endomyocardial biopsy. A negative NT proBNP rules out clinically meaningful cardiac involvement and may obviate the routine use of TTE in patients with a low clinical suspicion of cardiac amyloidosis.
Dispenzieri:Celgene, Takeda, Prothena, Jannsen, Pfizer, Alnylam, GSK: Research Funding. Gertz:Research to Practice: Consultancy; Physicians Education Resource: Consultancy; Ionis: Honoraria; celgene: Consultancy; spectrum: Consultancy, Honoraria; Teva: Consultancy; Amgen: Consultancy; Medscape: Consultancy; janssen: Consultancy; Alnylam: Honoraria; Abbvie: Consultancy; annexon: Consultancy; Apellis: Consultancy; Prothena: Honoraria. Lacy:Celgene: Research Funding. Dingli:Millennium Takeda: Research Funding; Millennium Takeda: Research Funding; Alexion Pharmaceuticals, Inc.: Other: Participates in the International PNH Registry (for Mayo Clinic, Rochester) for Alexion Pharmaceuticals, Inc.; Alexion Pharmaceuticals, Inc.: Other: Participates in the International PNH Registry (for Mayo Clinic, Rochester) for Alexion Pharmaceuticals, Inc.. Kapoor:Takeda: Research Funding; Celgene: Research Funding. Kumar:AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Roche: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract